Yeast GeneratorProbably the cheapest and still the best vessel you can use for a yeast generator is the two-liter soda bottle. If you can find one of those four-liter versions, that is even better. There are several factors that make the soda bottle a good choice. First off, it is designed to hold a solution of water with dissolved CO2 under pressure. This is important. The pressure that builds up in a yeast generator can be substantial. I would venture to say it is not lethal, but it certainly can make quite a mess if it fails and sprays sugar water and yeast all over your house. The cap and how to attach the tubing is another issue that has created much discussion. Most of these caps from soda bottles are made from polyethylene. Polyethylene does not readily bond with most glue. So gluing the tubing in place is not desirable. Leaks will occur, especially at the bond joint. Furthermore, since we're dealing with gasses, the seal must be airtight. The best all around solution is some mechanical means to attach tubing. Some type of bulkhead fitting is needed. Gas Delivery (tubing)Getting the gas to the tank water is the next consideration. Tubing should be selected based upon several factors. One is pressure retention, or the ability of tubing to retain its shape under pressure. As tubing is put under pressure, it should not expand in relation to its diameter. Also the tubing will need to be inert; meaning not break down over time due to chemical reaction with the CO2 gas internally or the air or water externally. This pretty much eliminates standard airline tubing used for fish tank aeration. Another consideration is flexibility. A good candidate for this application is silicon tubing. It does not react with CO2 as quickly, has good pressure retention characteristics and is very flexible. There is also special tubing designed specifically for carrying CO2 gas, and I would encourage spending the few extra dollars needed to use this. But silicon tubing will last for several years, and is in keeping with the cost savings approach DIY implies. It is also important that water is not allowed to run back down the line by suction or siphoning. This problem is easily remedied with the use of a check valve. Many check valves are available commercially. Several factors should be considered when selecting one. I would avoid choosing one made from metals. The caustic nature of CO2 gas, the high water vapor content of the gas (which usually contain carbonic acid), will cause a metal check valve to fail. Therefore it is important to choose a plastic valve or one designed specifically for CO2 applications. In addition, for the same reasons, I recommend avoiding the use of any metal components in the entire system. In pressurized tank systems, there is generally no liquids, or solids for that matter, to foul or corrode metal components. So the use of metal components is common in these systems. The same should not be assumed on a yeast based DIY system. Getting the gas dissolved in the waterThis is a topic that has received much attention on message boards, mailing list servers, and newsgroups over the years. And I think rightfully so! Many methods have been described on what the best way to dissolve the CO2 gas into the tank water. This is the critical point in determining the effectiveness of a DIY system and the reason why many feel that their experience with DIY systems was a bad one. Since the amount of CO2 available in a yeast system is limited by biological production, it is important to get most, if not all, the CO2 produced dissolved into the water. Skimp here, and you have wasted your time, not to mention CO2 gas. The simplest, and least effective, method is to run the tube into the tank and simply let the gas bubble into the tank, or through an air stone. I do not recommend this method at all. Since most of the CO2 gas simply rises to the surface and is lost. Next, many have suggested placing this tube at the inlet of a canister filter and allowing the impeller to munch up the gas. While it is effective in dissolving the gas, I do not like this method either, for two reasons. First, the CO2 bubbles can produce cavitations of the impellor, which could cause it to vibrate, making noise and possibly damage the mechanism. Second, some of the components in the impellor use rubber fittings, which could be broken down over time by the high concentrations of CO2 gas and carbonic acids present. 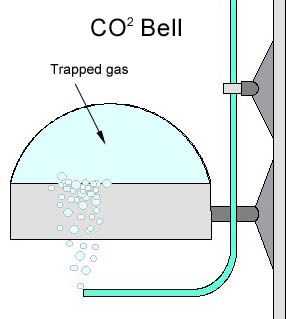 A better but slower method is the use of what is called a CO2 bell. Simply put, this is a hemispherical shaped vessel of some kind, inverted and the CO2 is allowed to fill up inside. The contact area of the gas is increased and passive diffusion of the gas is increased. The drawback of this is if the surface area is not high enough, so that diffusion rate exceeds gas production, the bell will fill with gas and any additional bubbles will run out the side and travel up to the surface and be lost. While this is a draw back, many aqaurists have have had reasonable sucess using this method of gas diffusion. These are also very simple to construct. Many have been constructed from cutting off the tops of one-liter soda bottles, petri dishes, cups, or any hemisphercal shaped object. I would recommend using a material or object that is transparent, to allow for easy viewing. A better but slower method is the use of what is called a CO2 bell. Simply put, this is a hemispherical shaped vessel of some kind, inverted and the CO2 is allowed to fill up inside. The contact area of the gas is increased and passive diffusion of the gas is increased. The drawback of this is if the surface area is not high enough, so that diffusion rate exceeds gas production, the bell will fill with gas and any additional bubbles will run out the side and travel up to the surface and be lost. While this is a draw back, many aqaurists have have had reasonable sucess using this method of gas diffusion. These are also very simple to construct. Many have been constructed from cutting off the tops of one-liter soda bottles, petri dishes, cups, or any hemisphercal shaped object. I would recommend using a material or object that is transparent, to allow for easy viewing.
Another method is a diffuser.  Two versions of diffusers exist. One is device that increases the time the bubble is in contact with the water. Usually by presenting the bubble with a long spiral course it has to travel. In the image to the right is one example of this type of spiral diffusion method, the Econo Aqualine 500 available from AquaBotanic, and others. The manufacturer claims, "The special construction allows a very high CO2 diffusion rate and automatically removes any false gasses. The reactor is sufficient for an aquarium up to 125 Gallons". This unit is mounted on the inside of the aquarium. Two versions of diffusers exist. One is device that increases the time the bubble is in contact with the water. Usually by presenting the bubble with a long spiral course it has to travel. In the image to the right is one example of this type of spiral diffusion method, the Econo Aqualine 500 available from AquaBotanic, and others. The manufacturer claims, "The special construction allows a very high CO2 diffusion rate and automatically removes any false gasses. The reactor is sufficient for an aquarium up to 125 Gallons". This unit is mounted on the inside of the aquarium. Another diffuser type is a glass diffuser. This is a device that increases the surface area of the CO2 gas by reducing the size of the bubbles substantially. This is a proven method and can be very effective in allowing all of your CO2 gas to be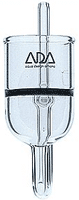 dissolved. In the image to the left is version of this type of diffuser made by Aqua Design Amano Nature Aquarium Goods, the company led by the legendary aquatic artist Takashi Amano. The gas is fed into the tube at the rear, brought down to the bottom and forced against the glass diffusser plate (the black line running in the middle). This plate has thousands of pores which the gas passes through, and once it has done this, the bubbles released through the top of the unit are extremely tiny. This all glass unit is probably the very best of its kind, and also very expensive since it is handmade in Japan. Other manufacturers make similar products. The only drawback of this method is that the plate, usually made of sintered glass, can clog and may need regular maintenance. Other than that singular drawback, this is a proven method of diffusion. The drawbacks of both versions is that their mechanical sophistication do not allow themselves to be easily homemade, and commercially produced products would have to be purchased. There are many commercially available choices, in a wide range of prices, so finding one that works in your budget would not be to difficult, if you decided on going this route. dissolved. In the image to the left is version of this type of diffuser made by Aqua Design Amano Nature Aquarium Goods, the company led by the legendary aquatic artist Takashi Amano. The gas is fed into the tube at the rear, brought down to the bottom and forced against the glass diffusser plate (the black line running in the middle). This plate has thousands of pores which the gas passes through, and once it has done this, the bubbles released through the top of the unit are extremely tiny. This all glass unit is probably the very best of its kind, and also very expensive since it is handmade in Japan. Other manufacturers make similar products. The only drawback of this method is that the plate, usually made of sintered glass, can clog and may need regular maintenance. Other than that singular drawback, this is a proven method of diffusion. The drawbacks of both versions is that their mechanical sophistication do not allow themselves to be easily homemade, and commercially produced products would have to be purchased. There are many commercially available choices, in a wide range of prices, so finding one that works in your budget would not be to difficult, if you decided on going this route. The best method, in my opinion, is the use of a forced reactor. 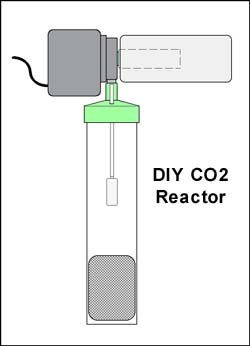 A forced reactor is one that can bring a large quantity of water to the gas. The previous methods are passive in this respect. In other words if circulation of the surrounding water is poor, then the diffusion may slow down due to super-staturation of the water immediatly around the diffuser. By forcing mass quantities of water to meet the gas, via a pump, and mixing it thouroughy the gas is forced into the water more quickly, and then circulated. In general a forced reactor is comprised simply of a water pump and a reaction chamber. Within the reaction chamber there is some course media to help churn up the gas and water, and increase contact time, as well as preventing bubbles of gas from escaping. This simplicity of design also lends itself very well to the DIY concept. The image to the right shows one example of a DIY Forced Reactor. It is simply comprised of a powerhead with prefilter, and gravel cleaning tube, a course filter pad, and an airstone. The cost to build this, if you where to buy all the parts, is inder $35US. More details on this reactor, and other construction projects, will be given at the end of this article. A forced reactor is one that can bring a large quantity of water to the gas. The previous methods are passive in this respect. In other words if circulation of the surrounding water is poor, then the diffusion may slow down due to super-staturation of the water immediatly around the diffuser. By forcing mass quantities of water to meet the gas, via a pump, and mixing it thouroughy the gas is forced into the water more quickly, and then circulated. In general a forced reactor is comprised simply of a water pump and a reaction chamber. Within the reaction chamber there is some course media to help churn up the gas and water, and increase contact time, as well as preventing bubbles of gas from escaping. This simplicity of design also lends itself very well to the DIY concept. The image to the right shows one example of a DIY Forced Reactor. It is simply comprised of a powerhead with prefilter, and gravel cleaning tube, a course filter pad, and an airstone. The cost to build this, if you where to buy all the parts, is inder $35US. More details on this reactor, and other construction projects, will be given at the end of this article. Additional Concepts and DesignsSince we are dealing with solids, liquids and gasses under pressure, it may also be a good idea to incorporate some features into a DIY system that improves both the reliability and saftey. Emergency pressure release valves and anti-clogging devices can be designed, built and utilized in that end. The construction section of this article details some additional concepts and designs in these areas.
More than you need to know about yeast.Yeastie the Beastie!Yeast is the primary ingredient in our DIY CO2 generators. Common baker yeasts are adequate for the needs of CO2 generators. But of course, I have to delve into the esoteric side of things. Yeast is a living organism and optimal living conditions give it the best opportunity to do what we need it to do, I had to touch upon this in this text. Also knowing there are as many strains of yeast as there are different algae, I have to touch on that also. It is also good to understand the biological processes involved here, and I will discuss this firstly. Theodor Schwann (1810-1882) named the yeast cells "Zuckerpilz" ("sugar fungus"), which later became Saccharomyces, the genus that most yeast belongs to. Yeasts, that belong to the kingdom Fungi, are classified as belonging to either of two major types: budding yeasts, named so because of the buds formed at the cell divisions, and fission yeasts that are rod-shaped and grow by elongation at their ends. Most yeast used is of the budding type. Although easily grown in culture media, each S. cerevisiae cell (the most common species for our purposes here) has a limited number of buddings of around 20. However, in a given culture only about half of the cells will have given rise to new cells, and only rarely does a cell give rise to as much as 20 new cells. Poisoning, mutations and heat are other factors that affect the viability of yeasts. Towards the end of fermentation many yeasts aggregate into clumps, a phenomenon known as flocculation. The process of flocculation is not completely understood, but it is believed to be mediated by bivalent ions such magnesium, calcium or manganese ions. Yeasts are probably the most researched organisms in microbiology. Entire scientific communities and disciplines have evolved surrounding this simple, single-cell fungi. If you want to blow your mind out one day, check out this link below. It is a list of researchers, their associated laboratories, and their research papers on the singular species Saccharomyces cerevisiae. This yeast has the distinction of not only being the one we generally use for our CO2 generators, but also being the first organism to have its entire genome (DNA) completely mapped in 1996. Yeast Labs and Research This is only for the brave of heart! Good luck! A more pragmatic description of the biology of yeast is given below. BIOLOGYYEAST: A living organism formed of only one cell. Each cell, which is a living being, of a spherical or ovoid form, is nothing but a tiny and simplified fungus the size of which does not exceed 6 to 8 thousandth of millimeter. Yeast, like any living organism, lives thanks to the presence of oxygen (aerobiosis); but it also has the remarkable ability of being adaptable to an environment deprived of air (anaerobiosis). To cope with its expenditure of energy, it can use different carbon substrates, mainly sugars: Glucose is the best favored food of Saccharomyces cerevisiae; Saccharose is immediately transformed into glucose and fructose by an enzyme which yeast has released; Maltose is the main endogenous substrate of French bread fermentation; it gets into the yeast cell thanks to a specific permease to be split afterwards into two molecules of glucose by maltase.
Many other sugars are also utilized. An interesting scientific work by Vern J. Elliot shows the utilization of sugars by yeast, and yields some insight into this question. If you look at the chart below you will see growth rates of yeast over time when fed by different sugars. Just to understand the chart, the reference of the test is as follows, (for you technically oriented folks out there) "... Plates (growth samples) were incubated at 28ºC and growth was determined at time zero and at approximately 24-h intervals by measuring absorbance at 630 nm with a microplate reader (Model ELx800UV, Bio-Tek Instruments, Winooski, VT)...". While this experiment tested some 250 different strains of yeast, and the chart above shows the strain labeled "isolate 59", a brief examination of the published paper shows that nearly all the strains showed similar results in terms of sucrose providing the highest growth rates. It can be reasoned that the yeast strains we use in our CO2 systems would have similar results. So what does this mean. Essentially, using less yeast and more cane sugar (sucrose), and allowing the yeast to grow and multiply will assure a longer lasting CO2 mixture. Conversly, CO2 quantity measured over time is another issue more related to use of specific mutant strains of yeast than type of sugar. Longevity of the yeast culture, due to toxic death, is also not related to type of sugar, but to alcohol levels. Acids play a much lesser role in this respect than popular belief, by the way. (More on this later). So, use of sucrose seems to be a better choice, other factors not withstanding, than other sugars. The conditions of oxygenation of the environment generate two types of metabolism: In AEROBIOSIS
When yeast is in presence of air, it produces, from sugar and oxygen, carbon dioxide, water and a great amount of energy. It is the metabolic process of respiration. In these conditions the oxidation of glucose is complete: Glucose + Oxygen —> Carbon dioxide + Water + Energy All the biochemical energy potentially contained in glucose is freed. Thanks to this energy, yeast ensures its life. But it can also use it to synthesize organically, that is to say start its growth and multiply. It will then have to find other nutritive elements in its environment, mainly nitrogen. In ANAEROBIOSIS When there is no oxygen available, yeast can nevertheless use sugars to produce the energy it needs to be maintained in life. Pasteur defined this metabolic process as being the fermentation process. Sugars are transformed into carbon dioxide and alcohol. The glucose oxidation is incomplete: Glucose —> Carbon dioxide + Alcohol + Energy The alcohol, which has been formed, still contains a great amount of energy. This constitutes only a part of the biochemical energy potentially present in glucose that was freed (about 20 times less than for respiration). It ensures a minimum level but does not enable yeast to multiply rapidly.
ANAEROBIOSIS is the process we use in our CO2 generators, although AEROBIOSIS would be preferred. Aerobiosis is preferred because it produces less alcohol, which is toxic to yeast at elevated relative level. But aerobiosis is also impractical for reasons you will see later. "God is Good" is the name which yeast was given in the early days of fermentation. This is prior to the time when Louis Pasteur, in the mid 1800's, discovered that, in fact there was actually a single cell microscopic organism responsible for the conversion of fermentable barley malt sugars into alcohol, carbon dioxide, and flavor compounds. As described by Gay-Lussac at the beginning of the nineteenth century, the chemical reaction of fermentation is as follows; C6H12O6 + Saccharomyces cerevisiae = 2C2H5OH + 2CO2
(Sugar plus yeast yields alcohol and carbon dioxide) |
The tail end of the formula is the thing we're looking for … CO2!!! Beverages including wine, fermented milk products, and mead from honey are some examples of what developed from spontaneous fermentation, which is now understood and managed in a scientific manner. Many of these organisms were discovered more by chance, than by design. Other types of yeast and bacteria are also utilized in various styles of beer and brewing beer like beverages. The following is a description of the many strains of yeast that are available for CO2 generation. Some are commonly available and inexpensive; some are harder to get and more expensive. The advantages and disadvantages of each type are explained. Bakers YeastBakers yeast (or Dutch Process yeast) is widely available at nearly every supermarket. It is dried active yeast. I like the term "mummy yeast" because it does seem to "rise" from the dead. Ouch! Bad pun, I know! Most of us know bakers yeast, popularized by companies like Fleishmann's. They manufacture little packets or you can buy 4oz. jars. It comes in several variations. Regular bakers yeast in 7-gram packets is by far the most common. Lately a new form known as "Bread Machine" yeast has appeared. This yeast is more tolerant of higher temperatures found when using these new automated bread machine thingies. Both work well in our application. The bread machine yeasts are available in 4 oz jars, which are more economical. Here are some detailed specifics on these types of yeast: The following information is typical for each type of bakers yeast, but may vary somewhat according to product and company: Compressed Yeast (also called cake, wet, and fresh yeast) Fleischmann's compressed yeast is available in supermarkets in 0.6 oz cakes, and Red Star compressed yeast is available in some supermarkets in 2 oz. cakes. It is found in the dairy or deli case. Compressed yeast is available to commercial bakers from a variety of companies in 1 and 2 pound packets. Compressed yeast has approximately 30% solids and 70% moisture content. It is highly perishable and must be stored at a uniformly low temperature (about 40º F) to prevent excessive loss of activity or gassing. Compressed yeast generally has a shelf life of approximately two weeks from its make or packaging date when kept at 73.3º F. (23ºC) At 32º-42º F. (0º - 5.5º C) compressed yeast loses approximately 10% of its gassing power over a 4-week period. At 45º F (7.2º C) yeast will lose 3-4% of its activity per week. At 95º F (35º C), one half of the gassing power is lost in 3-4 days. Once yeast starts to deteriorate or lose its fermentative activity, it does so quickly, losing almost all of its activity (autolysis) by the third week. It has, however, been shown that compressed yeast can be successfully stored for two months at 30º F. (-1º C). When this is done, good CO2 production can be made from yeast stored for two, but not three, months. To use compressed yeast, soften it in tepid water. Active Dry Yeast Fleischmann, Red Star, and SAF active dry yeast are available in supermarkets in ¼ oz (7 g) packets and/or 4 oz (113.4 g) jars. Active dry yeast is available to commercial bakers from a variety of companies in 1 and 2 pound, and 500 g packets. It also is available in these sizes to consumers at warehouse or club stores, and via mail order. Active dry yeast has approximately 92.0% solids and 8.0% moisture content. It is advisable to store active dry yeast in a cool, dry place that does not exceed 80ºF. The shelf life of "active dry yeast" stored at room temperature is approximately 2 years from its make date. Once opened, active dry yeast is best stored in an airtight container in the back of the refrigerator, where it will retain its activity for approximately 4 months. To rehydrate active dry yeast, blend one-part yeast with four parts lukewarm water, wait 10 minutes, and stir. Depending upon the particular product and company, lukewarm water ranges from 90º-115º F. Temperatures lower than 90º F and higher than 115º F should be strictly avoided. Instant Active Dry Yeast Fleischmann, Red Star, and SAF instant active dry yeast is available in supermarkets in ¼ oz (7 g) packets and/or 4 oz (113.4 g) jars. The Fleischmann product is marketed as RapidRise, the Red Star product is marketed as QUICK.RISE, and the SAF product is marketed as Gourmet Perfect Rise. Fleischmann also markets an instant active dry yeast named Bread Machine Yeast. Instant active dry yeast is available to commercial bakers in 1 and 2 pound, and 500 g packets. It also is available in these sizes to consumers at warehouse or club stores, and via mail order. Instant active dry yeast has 96.0% solids and 4.0% moisture content. It is advisable to store instant active dry yeast in a cool, dry place that does not exceed 80º F. The shelf life of instant yeast stored at room temperature is approximately 2 years from its make date. Once opened, instant active dry yeast can be stored in an airtight container in the back of the refrigerator, where it will retain its activity for approximately 4 months. To rehydrate instant active dry yeast, blend one-part yeast with five parts lukewarm water, wait 10 minutes, and stir.
It is worth noting that there is disagreement among the yeast companies as to whether or not active dry and instant active dry yeast should be frozen, and if in doing so the shelf life of the yeast is prolonged. The most convincing argument against freezing is that under normal conditions, there are temperature fluctuations in freezer units caused both by repeated opening and closing of the freezer door and, in contemporary freezer models, by the self-defrosting (freeze and thaw) cycle. These temperature fluctuations can cause damage to the yeast cell structure. One topic upon which there is agreement is that if active dry or instant active dry yeast has been refrigerated, and is going to be rehydrated in lukewarm water, it is best to allow the portion of yeast to be used to come to room temperature prior to blending it with the lukewarm water. Otherwise, temperature shock might damage the yeast cells. Unlike compressed yeast, which disperses in cold water without any problems, the temperature of the water during rehydration is important when working with dry yeast. When yeast is dried, the cell membrane becomes more porous. During rehydration, the membrane recovers. However, in the process of rehydration, some cell constituents are dissolved in the water used. The optimum water temperature for cell membrane restoration is 104º F. Warm water is effective in this process, because it leads to more rapid cell membrane recovery. Cold water impedes this process, because it slows membrane recovery and allows more cell constituents to leach out during the reconstitution process. The effect is not that great between 70º and 100º F, but at lower temperatures approximately one-quarter to one-half of soluble yeast cell constituents can be lost. This leaching action effects yeast activity in the following manner: Most yeast enzymes remain, but the soluble chemicals are depleted, and it is these chemicals that promote enzyme activity. Brewing YeastThese are specific strains of yeast that are used in the brewing of beer. There is a wide variety of brewers yeasts bred specifically for different types of beer, and is what makes most brands taste different by the way. It's not the "…clear mountain water" or "…the loving hands of the brew master". It's the bugs they put in it! Use a different bug; get a different tasting lager or ale. Saccharomyces cerevisiae, and Saccharomyces uvarum are the genus and species of ale, and lager yeast respectively. These are the primary types of yeast cultures, which produce most of the world's beers. The Ale yeast is a specialized strain of S. cerevisiae, which adapts better to higher alcohol levels. Most of these are live cultures in liquid form, and do not require the rehydration process used with dry yeasts. Wine or Champagne YeastThese are very specialized yeast strains that do different things, like soften the wine's acidity or absorb tannins lightly. This is accomplished by the release of enzymes specific to this strain of S. cerevisiae. In addition, they also can ferment at a wide range of temperatures and can tolerate the highest alcohol and acid levels, which is toxic to most yeast. This is an important point for our application. Another benefit side effect is that this yeast has a tendency of settling towards the bottom of a culture, or it is said to be a bottom flocculent. Bakers and Ale yeasts are top flocculants, which is that gooey, tan head on the top the sugar water you see when using bakers yeast. Champagne yeasts usually do not have this build up of yeast at the surface. Therefore they can help reduce a common problem with DIY CO2 systems, the clogging of the airlines, and raw yeast getting pumped into the tank. Some of the best yeasts, discovered in my testing for our application, are sold under the brand names "Pasteur Champagne" and "Eau de Vie", from Wyeast Labs, Inc. in Mt. Hood, Oregon. Again, as with brewers yeast, most of these are sold as live cultures in liquid form, and do not require the rehydration process used with dry yeasts. What are the advantages of the more esoteric yeast for DIY CO2?Right off, I will say that you can certainly use the common bakers yeast with great success. It is more than adequate. But there are certain factors where you may want to optimize the performance of your system. One downright frustrating thing about DIY CO2 is the maintenance and replenishment of the mixture. You have to change your mixture every 7-14 days, depending on how well your particular formula works. Fourteen days seems to be the limit for most yeast mixtures in a two-liter bottle when using bakers yeast. This is due to the fact that the alcohol levels reach a point where it kills the yeast cells, even if it hasn't used up all the sugar. The general consensus has been that it is the rise in acid levels that kills off the yeast. But this is probably not true. One way that has been proposed is to add baking powder as a buffer to the mixture to regulate the acids, but this does little to effect the alcohol levels. Oddly it is not the acids that are problematic. Yeast can generally deal with acidic levels to a point, as you will see below. Yeast Tolerance to Acidity Yeast exhibits a considerable tolerance to extremes of pH, being able to maintain an active fermentation in a 5% glucose solution in the pH range of 2.4 to 7.4, but ceasing activity at pH 2.0 or pH 8.0. For optimum results, good practice dictates that the pH of the fermenting medium be maintained within the range of about 4.0 to 6. A drop of more than 50% in fermentative activity has been observed at pH 3.5. More gradual declines in yeast activity were encountered at higher pH levels, with measurable effects showing up at pH values over 6.0. The explanation for the yeast's ability to maintain a relatively constant activity over a 100-fold change in hydrogen ion concentration (pH 4 to 6) is found in the fact that the pH of the cell interior of the yeast remains quite constant at about pH 5.8, regardless of any relatively wide pH variations in the fermenting medium. The enzymes involved in fermentation thus operate in an optimum pH environment within the yeast cell that is largely unaffected by external changes in pH.
Conversely, sodium ions are also toxic to yeast, so once the sodium biphosphate has been broken down by the acids, the free sodium ions tend to kill off more yeast cells. So this method is only a transparent fix to the yeast kill-off. The logical alternative is to find strains of yeast more resistant to high alcohol levels, since alcohol appears to be the true killer. The apparent regulation by buffering with baking powder is probably due to the issue of sodium slowing the reproduction process, thereby slowing the consumption of sugar by limiting the population of living yeast cells. While this extend the life of the mixture, it also reduces the CO2 output over the lifespan of the mixture. This is a result of reduced, or at least controlled, yeast cell population. Brewers yeast is one step in the right direction. Strains of Saccharomyces cerevisiae brewers yeast, commonly referred as Ale Yeast, is a good choice for this. It is more tolerant to higher alcohol levels and should provide a longer lasting mixture; usually by about 4-6 days longer than the bakers yeast strain. It is also seems to be more tolerant of sodium. Using Ale Yeast in your mixture can yield a longer lasting mixture. Champagne Yeast is tolerant of the highest alcohol levels, and wider temperature ranges. Another side effect is that its metabolism seems to be in hyper-speed, producing nearly twice as much CO2 as other strains. It also ferments well at average room temperatures. This makes it the perfect yeast strain (and the most expensive) for our applications. I have had mixtures with this strain, very carefully prepared aseptically, last strongly for nearly 24 days. Now for the downside. Costs are very high with these esoteric yeasts. The cost is nearly 3-5 times more expensive as common bakers yeast. Also, these yeasts work better if inoculated into your sugar water when they are alive, which is the form they are purchased in. This makes storing them difficult. The manufacturers makes these available to home vintner's, and are prepared in much larger quantities than we would use. Anything left over last only for a very short period, and is difficult to store and keep viable. Finally, it should be noted that there is also an inverse relation between the amount of yeast and fermentation time. Thus, a reduction in the amount of yeast will result in longer fermentation times, while an increase in the amount of yeast will shorten them. We'll talk about this more in the section on mixtures. Yeast is hardy, yeast is intolerant.Now that's an oxymoron if I ever saw one. But that's the nature of the yeast. It can withstand drying, pounding, skimming, centrifugal forces, replicates itself easily; yet in the wrong situation it will crash faster than a CO2 injected tank with a 1dkH when the mixture runs out. Opps…got ahead of myself there. One of the most important issues to remember when using yeast in fermentation is cleanliness. Yeast does not compete well against bacteria, so it is important to keep things as close to sterile as possible. One excellent and simple way to deal with this is as follows. Note of Caution: Be very careful with this, since very bad burns can be had here. Thoroughly rinse out your two-liter bottle with hot water; use no soap or detergent. Keep an extra bottle cap handy. Boil the water you plan to use, and place this extra cap in the water to sterilize it. Pour the boiling water (use a funnel) into your two-liter bottle. While it is still ripping hot, add your sugar, and use the cap you boiled clean, and cap the bottle tightly. Shake well until most of the sugar dissolves. This sterilizes the bottle, water, and the sugar. This is what they call an aseptic preparation. Do not uncap this until you let the water cool to room temperature and are ready to add the yeast. If you plan to use dry yeast you should activate the culture first. As discussed previously, yeast needs to start in an aerobic environment first, so it can then readily adapt to the anaerobic conditions in our little fermentation factory. Many folks who omit this step believe they are creating this situation with just the action of pouring the mixture into their bottle. But they also do not realize that much of the yeast they use dies, because many of the yeast cells could not complete the aerobic phase of its life before the conditions change to anaerobic. This step insures all the yeast is already aerobically active and working before it is placed in the generator. The time it takes for the generator to begin producing pressurized CO2 is significantly reduced by this following step. These yeasts also need to be rehydrated properly, as also previously mentioned, so as to not damage the yeast cell walls. I take my measure of yeast, add a small quantity of tepid water, 100º-115ºF, (not hot), and stir it up in a little cup with a fork. Stir the mixture until the yeast in no longer in clumps, but instead a smooth creamy tan liquid. Now here's the part everyone forgets, add a few pinches of sugar and vigorously mix the yeast liquid up making lots of bubbles. You want to get oxygen in there to get the yeast going. Once this is done, let the mixture stand for about ten minutes. Then take your funnel in hand, open the aseptic bottle you prepared, and pour in your yeast culture. Now granted this isn't a perfectly sterile method, but by reducing the chances of bacteria getting in on the outset, the yeast mixture will prevail quicker and last that much longer. More information about specific mixtures, formulas, and capacities of systems will be given in the next section of this article. Authors Final Notes on YeastIn preparing this article, I have conducted research into yeast and dove head first into the scientific data available from researchers. To date I have not been able find data on any specific strain mutated for its ability to produce CO2 gas exclusively, while it appears there has been some strains developed. Generally, mutant strains of yeast are selectively bred for controlled production of by-products like alcohol and carbon dioxide. Can yeasts be improved for our purposes? Most likely work will continue on this process for as long as there are chemists, and geneticists interested in yeasts. One of the more interesting new research areas in this domain is the work on recombinant-DNA technology as it pertains to the development of newer yeast strains. This work has led to changes in formulation, ingredients and processing conditions. Some of this work has led to new strains of yeast that are more resistant to stress, produce more proteins, and more carbon dioxide. Some discussion exists on the genes in the HTX group and the role they play in the cell utilizing glucose in various stages of it life. In the yeast Saccharomyces cerevisiae, the Snf1/AMP-activated protein kinese family is particularly important for the response to glucose deprivation, and this kinase regulates genomic transcription, metabolic activity, and developmental processes such as invasive growth. These genetic research results show, in effect, the potential for a mutant strain that is longer lasting since it could be designed to utilize less glucose, or require less glucose to produce CO2 in useful quantities for our purposes here. If there is any other specific scientific data on this, I would be interested in seeing it.
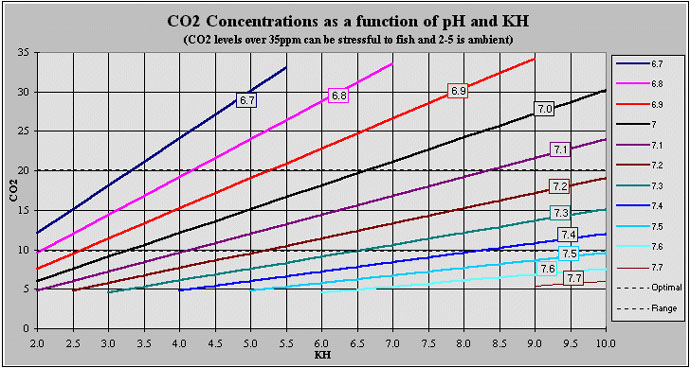
Guidelines for Mixtures and CapacitiesIt is important to understand that the yeast/sugar/water mixture is not a precise science. You will have to experiment to find what works best for your situation. I will give some suggestions in this section on formula's based upon the scientific data presented above and my own personal experience. Mixture FormulasFor two-liter bottles: 2 cups water 2 cups Sucrose (cane sugar) ¼ teaspoon Fleischmann's Active Dry Yeast ¼ cup tepid (ideally 104ºF) water The yeast should rehydrated first in the tepid water. The aseptic method mentioned previously should be used.
This is the easiest formula, using the most widely available ingredients. This mixture can last up to approximately 16 days, if the aseptic method is used. An improved formula is: This is a more costly mixture. Since this yeast is a liquid live yeast, (refered to as pitchable) it does not need to be rehydrated. This yeast is more tolerant to higher alcohol levels, therefore this mixture can last up to approximately 22 days. It also has a more consistant output of gas over time. Tips on MixturesYou should experiment on your mixtures. Everybody has different water, with its own unique chemistry. And this influences the performance of your mixture. So try slight modifications of the yeast/sugar/water ratios till you find what lasts the longest for your situation. There is evidence that yeast mixtures last longer if you decrease the amount of yeast, and conversely last shorter periods if more yeast is used. Lower yeast also means less CO2 produced per minute, but produce more consistantly over time. Higher yeast levels will cause an initial higher burst of CO2 production with a gradually declining production over time. Keep this in mind while experimenting. There also has been some discussion on adding other nutrients to the mixture to help the yeast grow and multiply. This is an area I have not researched directly, but may be of interest. Nitrogen seems to be a nutrient that yeast utilizes. Unfortunately the research I have gleened showed that nitrogen is used by yeast only when it is in an aerobic environment, not the anaerobic environment we are putting it in. I have not seen any direct research that showed any nutrient other than sugars are used by yeast in anaerobiosis. Speaking of aerobiosis, as shown before this reaction only produces CO2 and water as by-products. Alchohol is not produced. So why not just produce an aerobic environment? This would involve injecting oxygen (O2) into the mixture chamber. Now one could aerate the mixture to do this, but of course this would be sent out into the system that is supposed to dissolve only CO2 gas. The problem is obviously a matter of either injecting an air mixture with elevated CO2 levels into your tank, or choosing a pure CO2 gas to be dissolved. The latter is what is prefered, and in fact essential for a CO2 system to work from a practical concern. So unfortunately we still will have to deal with alcohol as a by-product, and deal with it accordingly. System CapacitiesA good CO2 system will provide enough gas to supply a given amount of water to reach the idealized level of 15ppm of dissolved CO2. As a rule of thumb, each two-liter bottle will provide enough gas for up to 30 gallons of tank water, assuming you use an efficient method of mixing the gas and tank water. It is important to remember when using CO2 injection in a freshwater planted aquarium that doing so reduces the pH in the water. Some of the CO2 dissociates and forms Carbonic Acid. This results in a lowering of pH due to the addition, or more accurately put, the creation of acids in the tank water. To prevent wide fluctuations in pH over the life of your mixtures it is important to make sure the buffering capacity of your tank is high enough. This is determined by measuring the carbonate hardness of your tank water. This is refered to as your waters kH. A good kH for most planted tanks is around 6dkH. This will ensure your pH doesn't swing to abruptly over the life span of your mixture. Interestingly, you can use this relationship of carbonate hardness and pH to determine you CO2 level in your tank. If you have fresh and accurate test kits, one for pH and another for kH, you can use the chart below to determine your CO2 levels. Chart derived by George Booth, rendered by Jeff Dietsch
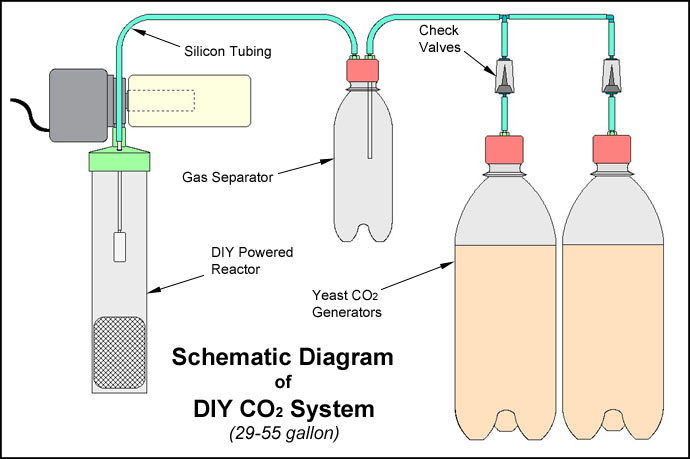
Construction ProjectsOkay, here's the fun part. Actually making a DIY CO2 System of your own. On average, a DIY CO2 system for a 55 gallon tank, like the one depicted at the beginning of this article, would cost about $50 to make, complete! And that includes the soda. Remember, that's probably about a quarter of the price of a complete pressurized system with a diffuser of some kind. While the pressurized system is the best way to accomplish CO2 injection, the DIY method will provide very excellent performance, costs substancially less money, and can be even fun to build if you're handy. There is nothing like the pride and satifaction of knowing that you "Did It Yourself"! The Perfect Two-Liter Bottle Generator While using a two-liter soda bottle for a generator is not a new idea, one of the downfalls of most attempts has been in connecting the tubing to the bottle cap. Most caps are manufactured from a material known as polyethylene. It is used because it wears well, is resistant to bacterial growth, and is resistant to acids. It also has a excellent ability to deal with pressure. In addition to being a good candidate for a soda bottle cap, these same charecteristics make it ideal for our application. But unfortunately, polyethylene is also difficult to bond with most adhesives. While using a two-liter soda bottle for a generator is not a new idea, one of the downfalls of most attempts has been in connecting the tubing to the bottle cap. Most caps are manufactured from a material known as polyethylene. It is used because it wears well, is resistant to bacterial growth, and is resistant to acids. It also has a excellent ability to deal with pressure. In addition to being a good candidate for a soda bottle cap, these same charecteristics make it ideal for our application. But unfortunately, polyethylene is also difficult to bond with most adhesives.
Most instructions for using soda bottles for generators advise drilling a small hole, and gluing the airline in place. This has been the weak link in most DIY generators. The poor bonding capabilities of the cap always lead to seal failure, and consequent leaks. An improved way of dealing with this would be to have some type of mechanical seal, and a bulkhead type fitting for the tubing to connect to. This would be better engineering practice. To the left is a diagram showing the completed two-liter bottle generator with the bulkhead fitting in place. One of my other hobbies is RC airplanes, specifically jets! This little device is a nylon bulkhead fitting for engine fuel. It is designed for use with silicon  tubing just like the type we use with CO2 systems. It screws in tightly into the hole in the two-liter bottle cap, makes an airtight mechanical seal, and makes removing the tubing from the bottle a breeze when you change your mixture. tubing just like the type we use with CO2 systems. It screws in tightly into the hole in the two-liter bottle cap, makes an airtight mechanical seal, and makes removing the tubing from the bottle a breeze when you change your mixture. Here is a link to a place where you can purchase this online: Tower Hobbies It's manufactured by Fourmost Products, Oregon, and sells for around $3.75US. If you have a very good hobby shop nearby, you may be able to get it there. You get two fittings in the package. I love these things; they made doing mixture changes and worn cap replacement a breeze! |







 A better but slower method is the use of what is called a CO2 bell. Simply put, this is a hemispherical shaped vessel of some kind, inverted and the CO2 is allowed to fill up inside. The contact area of the gas is increased and passive diffusion of the gas is increased. The drawback of this is if the surface area is not high enough, so that diffusion rate exceeds gas production, the bell will fill with gas and any additional bubbles will run out the side and travel up to the surface and be lost. While this is a draw back, many aqaurists have have had reasonable sucess using this method of gas diffusion. These are also very simple to construct. Many have been constructed from cutting off the tops of one-liter soda bottles, petri dishes, cups, or any hemisphercal shaped object. I would recommend using a material or object that is transparent, to allow for easy viewing.
A better but slower method is the use of what is called a CO2 bell. Simply put, this is a hemispherical shaped vessel of some kind, inverted and the CO2 is allowed to fill up inside. The contact area of the gas is increased and passive diffusion of the gas is increased. The drawback of this is if the surface area is not high enough, so that diffusion rate exceeds gas production, the bell will fill with gas and any additional bubbles will run out the side and travel up to the surface and be lost. While this is a draw back, many aqaurists have have had reasonable sucess using this method of gas diffusion. These are also very simple to construct. Many have been constructed from cutting off the tops of one-liter soda bottles, petri dishes, cups, or any hemisphercal shaped object. I would recommend using a material or object that is transparent, to allow for easy viewing. Two versions of diffusers exist. One is device that increases the time the bubble is in contact with the water. Usually by presenting the bubble with a long spiral course it has to travel. In the image to the right is one example of this type of spiral diffusion method, the Econo Aqualine 500 available from AquaBotanic, and others. The manufacturer claims, "The special construction allows a very high CO2 diffusion rate and automatically removes any false gasses. The reactor is sufficient for an aquarium up to 125 Gallons". This unit is mounted on the inside of the aquarium.
Two versions of diffusers exist. One is device that increases the time the bubble is in contact with the water. Usually by presenting the bubble with a long spiral course it has to travel. In the image to the right is one example of this type of spiral diffusion method, the Econo Aqualine 500 available from AquaBotanic, and others. The manufacturer claims, "The special construction allows a very high CO2 diffusion rate and automatically removes any false gasses. The reactor is sufficient for an aquarium up to 125 Gallons". This unit is mounted on the inside of the aquarium.  dissolved. In the image to the left is version of this type of diffuser made by Aqua Design Amano Nature Aquarium Goods, the company led by the legendary aquatic artist Takashi Amano. The gas is fed into the tube at the rear, brought down to the bottom and forced against the glass diffusser plate (the black line running in the middle). This plate has thousands of pores which the gas passes through, and once it has done this, the bubbles released through the top of the unit are extremely tiny. This all glass unit is probably the very best of its kind, and also very expensive since it is handmade in Japan. Other manufacturers make similar products. The only drawback of this method is that the plate, usually made of sintered glass, can clog and may need regular maintenance. Other than that singular drawback, this is a proven method of diffusion. The drawbacks of both versions is that their mechanical sophistication do not allow themselves to be easily homemade, and commercially produced products would have to be purchased. There are many commercially available choices, in a wide range of prices, so finding one that works in your budget would not be to difficult, if you decided on going this route.
dissolved. In the image to the left is version of this type of diffuser made by Aqua Design Amano Nature Aquarium Goods, the company led by the legendary aquatic artist Takashi Amano. The gas is fed into the tube at the rear, brought down to the bottom and forced against the glass diffusser plate (the black line running in the middle). This plate has thousands of pores which the gas passes through, and once it has done this, the bubbles released through the top of the unit are extremely tiny. This all glass unit is probably the very best of its kind, and also very expensive since it is handmade in Japan. Other manufacturers make similar products. The only drawback of this method is that the plate, usually made of sintered glass, can clog and may need regular maintenance. Other than that singular drawback, this is a proven method of diffusion. The drawbacks of both versions is that their mechanical sophistication do not allow themselves to be easily homemade, and commercially produced products would have to be purchased. There are many commercially available choices, in a wide range of prices, so finding one that works in your budget would not be to difficult, if you decided on going this route. A forced reactor is one that can bring a large quantity of water to the gas. The previous methods are passive in this respect. In other words if circulation of the surrounding water is poor, then the diffusion may slow down due to super-staturation of the water immediatly around the diffuser. By forcing mass quantities of water to meet the gas, via a pump, and mixing it thouroughy the gas is forced into the water more quickly, and then circulated. In general a forced reactor is comprised simply of a water pump and a reaction chamber. Within the reaction chamber there is some course media to help churn up the gas and water, and increase contact time, as well as preventing bubbles of gas from escaping. This simplicity of design also lends itself very well to the DIY concept. The image to the right shows one example of a DIY Forced Reactor. It is simply comprised of a powerhead with prefilter, and gravel cleaning tube, a course filter pad, and an airstone. The cost to build this, if you where to buy all the parts, is inder $35US. More details on this reactor, and other construction projects, will be given at the end of this article.
A forced reactor is one that can bring a large quantity of water to the gas. The previous methods are passive in this respect. In other words if circulation of the surrounding water is poor, then the diffusion may slow down due to super-staturation of the water immediatly around the diffuser. By forcing mass quantities of water to meet the gas, via a pump, and mixing it thouroughy the gas is forced into the water more quickly, and then circulated. In general a forced reactor is comprised simply of a water pump and a reaction chamber. Within the reaction chamber there is some course media to help churn up the gas and water, and increase contact time, as well as preventing bubbles of gas from escaping. This simplicity of design also lends itself very well to the DIY concept. The image to the right shows one example of a DIY Forced Reactor. It is simply comprised of a powerhead with prefilter, and gravel cleaning tube, a course filter pad, and an airstone. The cost to build this, if you where to buy all the parts, is inder $35US. More details on this reactor, and other construction projects, will be given at the end of this article.
 While using a two-liter soda bottle for a generator is not a new idea, one of the downfalls of most attempts has been in connecting the tubing to the bottle cap. Most caps are manufactured from a material known as polyethylene. It is used because it wears well, is resistant to bacterial growth, and is resistant to acids. It also has a excellent ability to deal with pressure. In addition to being a good candidate for a soda bottle cap, these same charecteristics make it ideal for our application. But unfortunately, polyethylene is also difficult to bond with most adhesives.
While using a two-liter soda bottle for a generator is not a new idea, one of the downfalls of most attempts has been in connecting the tubing to the bottle cap. Most caps are manufactured from a material known as polyethylene. It is used because it wears well, is resistant to bacterial growth, and is resistant to acids. It also has a excellent ability to deal with pressure. In addition to being a good candidate for a soda bottle cap, these same charecteristics make it ideal for our application. But unfortunately, polyethylene is also difficult to bond with most adhesives.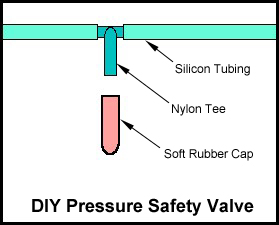 and some soft plastic or rubber cap. The part of the tee for the cap must have its barb carefully sanded off with emery cloth until it is smooth. It must be sanded down in such a way where the cap fits on snugly so as to not leak, but also pop off if the pressure builds up high enough. This has to be done thru careful trial and error. I take a bottle of seltzer, put the special cap on it for the generator and fit the tee on with the cap and the other side of the tube blocked or plugged. I then shake the seltzer bottle till some pressure builds and up and observe the point at which the cap blows off. It takes some time and patience to make one these work right. If you sand down too much and the cap is not snug enough it will blow to early, so you have to then start over with a new tee and test again. The whole process of making one of these do-hickey's is a little bit tweaky, so if do not have the patience to sit down and carefully craft one these I would recommend not doing it. It will, if not made properly, work when you don't want it to and cause more trouble. This unit should be placed between what runs into the tank and a Gas Separator. Which lead me to the following ...
and some soft plastic or rubber cap. The part of the tee for the cap must have its barb carefully sanded off with emery cloth until it is smooth. It must be sanded down in such a way where the cap fits on snugly so as to not leak, but also pop off if the pressure builds up high enough. This has to be done thru careful trial and error. I take a bottle of seltzer, put the special cap on it for the generator and fit the tee on with the cap and the other side of the tube blocked or plugged. I then shake the seltzer bottle till some pressure builds and up and observe the point at which the cap blows off. It takes some time and patience to make one these work right. If you sand down too much and the cap is not snug enough it will blow to early, so you have to then start over with a new tee and test again. The whole process of making one of these do-hickey's is a little bit tweaky, so if do not have the patience to sit down and carefully craft one these I would recommend not doing it. It will, if not made properly, work when you don't want it to and cause more trouble. This unit should be placed between what runs into the tank and a Gas Separator. Which lead me to the following ...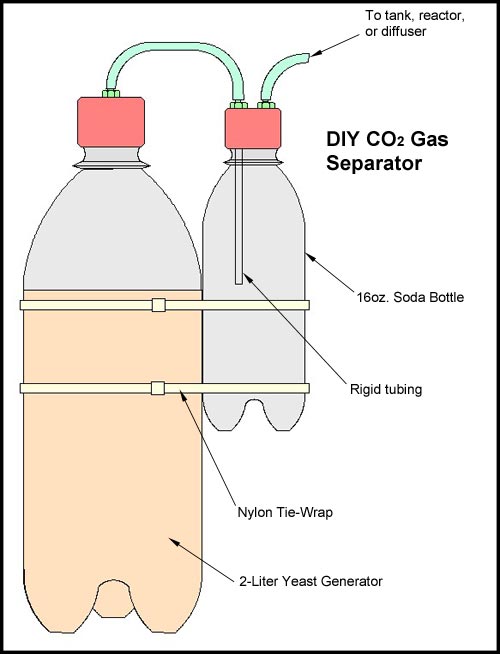
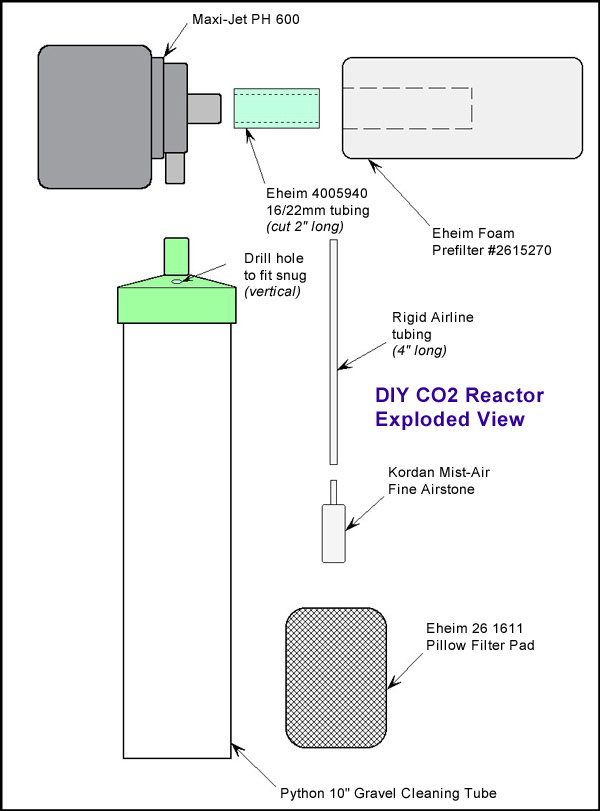
 It delivers a flow rate of about 160gph, which in theory would pump the water of a 55 gallon tank twice every hour through the reactor chamber. Most of the Maxi-Jet's supplied accessories are unused except the suction cups used to attach it to the tank wall. The pump itself will be used unmodified for this application. The Maxi-Jet 600 PH pump and power head includes an innovative triple-suction cup mount which is sufficient to hold the entire reactor in place inside your tank. It is a fully submersible powerhead and the only items that need to be fed out of the tank will be the AC power cord for the powerhead, and the CO2 supply line.
It delivers a flow rate of about 160gph, which in theory would pump the water of a 55 gallon tank twice every hour through the reactor chamber. Most of the Maxi-Jet's supplied accessories are unused except the suction cups used to attach it to the tank wall. The pump itself will be used unmodified for this application. The Maxi-Jet 600 PH pump and power head includes an innovative triple-suction cup mount which is sufficient to hold the entire reactor in place inside your tank. It is a fully submersible powerhead and the only items that need to be fed out of the tank will be the AC power cord for the powerhead, and the CO2 supply line.  No-Spill water changer system. These tubes cost about $10US. This particular one is the 10" version. The tube is a clear plastic and the flange at the top is a softer more pliable plastic. Take this flange and remove it from the tube. On the top, halfway between the outer edge and the hole for the tubing, drill a small hole to fit the rigid tube that will be inserted for the airstone and the inlet of CO2. This can be done by twisting a drill bit between your fingers. Make sure this hole is drilled perfectly parallel to the clear plastic tube walls. The diameter of the hole will be determined by the particular rigid tubing you use. Make sure the hole is slightly smaller than the outer diameter of the tubing chosen, so as to assure a very tight fit. More on this later.
No-Spill water changer system. These tubes cost about $10US. This particular one is the 10" version. The tube is a clear plastic and the flange at the top is a softer more pliable plastic. Take this flange and remove it from the tube. On the top, halfway between the outer edge and the hole for the tubing, drill a small hole to fit the rigid tube that will be inserted for the airstone and the inlet of CO2. This can be done by twisting a drill bit between your fingers. Make sure this hole is drilled perfectly parallel to the clear plastic tube walls. The diameter of the hole will be determined by the particular rigid tubing you use. Make sure the hole is slightly smaller than the outer diameter of the tubing chosen, so as to assure a very tight fit. More on this later.  recommend in this application. One of the things I have found with airstones in general when using them with CO2, is that the acidic environment tend to make airstones disintegrate quickly. The Kordon Air-Mist stones do not exhibit this problem. Also the fine airstone produces very small CO2 bubbles. There is a medium airstone from Kordon that produces bubbles the same size in fresh water, but the added back pressure in the fine stone helps in the regulation of yeast based DIY CO2 generators. It sells for about $3US.
recommend in this application. One of the things I have found with airstones in general when using them with CO2, is that the acidic environment tend to make airstones disintegrate quickly. The Kordon Air-Mist stones do not exhibit this problem. Also the fine airstone produces very small CO2 bubbles. There is a medium airstone from Kordon that produces bubbles the same size in fresh water, but the added back pressure in the fine stone helps in the regulation of yeast based DIY CO2 generators. It sells for about $3US.  It is a course, stretchable, nylon webbing tube rolled-up into itself to form a pad. Take this and insert it into the bottom of the gravel tube. Any material that is like course webbing will work, this was simply convenient for me. Do not use a foam pad, unless you can see through it easily. Foam pads will clog, mostly with nitrifying bacterial growth, which will inhibit out-flow of the reactor. This may lead to excessive back pressure problems on the CO² supply, especially when yeast generators are used. So use something very course, and that fits snuggly into the reactor chamber. Otherwise, it may be blown out of the bottom of the tube. This Eheim pad works great if you can find it. I will give a list of online dealers who have it (and the other parts) in stock, as of this writing.
It is a course, stretchable, nylon webbing tube rolled-up into itself to form a pad. Take this and insert it into the bottom of the gravel tube. Any material that is like course webbing will work, this was simply convenient for me. Do not use a foam pad, unless you can see through it easily. Foam pads will clog, mostly with nitrifying bacterial growth, which will inhibit out-flow of the reactor. This may lead to excessive back pressure problems on the CO² supply, especially when yeast generators are used. So use something very course, and that fits snuggly into the reactor chamber. Otherwise, it may be blown out of the bottom of the tube. This Eheim pad works great if you can find it. I will give a list of online dealers who have it (and the other parts) in stock, as of this writing.